Development of immortalized mouse aortic endothelial cell lines
Vascular Cell. 2014;
Received: 30 December 2013 | Accepted: 10 March 2014 | Published: 1 April 2014
Vascular Cell ISSN: 2045-824X
Abstract
Background
The understanding of endothelial cell biology has been facilitated by the availability of primary endothelial cell cultures from a variety of sites and species; however, the isolation and maintenance of primary mouse aortic endothelial cells (MAECs) remain a formidable challenge. Culturing MAECs is difficult as they are prone to phenotypic drift during culture. Therefore, there is a need to have a dependable
Methods
Here, we developed an effective method to prepare immortalized MAEC (iMAEC) lines. Primary MAECs, initially isolated from aortic explants, were immortalized using a retrovirus expressing polyoma middle T-antigen. Immortalized cells were then incubated with DiI-acetylated-low density lipoprotein and sorted via flow cytometry to isolate iMAECs.
Results
iMAECs expressed common markers of endothelial cells, including PECAM1, eNOS, VE-cadherin, and von Willebrand Factor. iMAECs aligned in the direction of imposed laminar shear and retained the ability to form tubes. Using this method, we have generated iMAEC lines from wild-type and various genetically modified mice such as p47phox-/-, eNOS-/-, and caveolin-1-/-.
Conclusion
In summary, generation of iMAEC lines from various genetically modified mouse lines provides an invaluable tool to study vascular biology and pathophysiology.
Keywords
MAEC Endothelial cells Shear stress p47phox eNOS cav1Introduction
Extensive research supports the notion that several common vascular diseases are, in part, a consequence of endothelial responses to shear stress from blood flow; i.e., that prolonged endothelial activation leads to dysfunction, which is an early, preclinical component of vascular disease [1, 2]. Unfortunately, it is difficult to access vascular tissue directly
EC from different origins and species have been successfully cultured for several decades [5, 6]. The most common human primary ECs used in culture are human umbilical cord vein endothelial cells (HUVEC) [7], human aortic endothelial cells (HAEC) [8], human coronary artery endothelial cells (HCAEC) [9], and microvascular ECs [10, 11]. In addition, ECs have been isolated from various species, such as bovine aortic endothelial cells (BAEC) [12], pig aortic endothelial cells (PAEC) [13] and mouse EC [3, 14–27]. Due to numerous transgenic mouse lines, the isolation and culture of mouse ECs is of particular interest. Several studies have developed methods for isolation of primary mouse aortic endothelial cells (MAEC) for
In this study, we have developed an effective method that enabled us to generate several iMAEC lines, including iMAEC from wild-type mice (iMAEC-WT), eNOS knockout mice (iMAEC-eNOS), caveolin-1 knockout mice (iMAEC-cav), and p47phox knockout mice (iMAEC-p47). We carried out extensive characterization to confirm that these iMAEC lines maintain endothelial phenotype and functional characteristics during culture.
Methods
Mice
Mouse aortic endothelial cells (MAEC) were isolated from the thoracic and abdominal aortas of various control and knockout mouse lines. Wild-type C57Bl/6 and p47phox knockout mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Caveolin-1 (cav1) knockout mice were kindly provided by Dr. Marek Drab (Max Planck Institute for Molecular Cell Biology and Genetics, Dresden, Germany). eNOS knockout mice were kindly provided by Dr. Mark C. Fishman and Dr. Paul Huang (Cardiovascular Research Center, Harvard Medical School, Charlestown, MA). All animals were maintained according to the approved Institutional Animal Care and Use Committee protocol by Emory University.
Primary MAEC isolation
Initially, 4-week-old male mice were used for MAEC isolation. Each mouse was sacrificed by CO2 asphyxiation and cleaned using 70% ethanol. The abdominal and thoracic cavities were opened and the mouse was perfused, via the left ventricle, with 3-4 mL of sterile heparinized (10 U/mL) 1X Hank's buffered salt solution (HBSS, Cellgro). Most organs were removed except for the thoracic/abdominal aorta, which was left intact. Perivascular fat and adventitia were removed from the ventral side of the aorta. To decrease contamination of medial smooth muscle cells, the cleaned aortas were perfused with HBSS containing 0.5% Triton X-100 for 5 minutes. The aorta was then dissected out, rinsed 5 times with fresh HBSS, and placed in a sterile dish of cold HBSS. The aorta was then cut into small rings (~1 mm length) using a sterile scalpel. Then, each aortic ring was opened and was carefully laid on a collagen gel bead with the endothelial cells directly facing the gel. The collagen gel consisted of type I collagen (Bio-Rad) diluted with EGM2-MV (Lonza) to a final concentration of 1.75 mg/mL. Each collagen gel bead was prepared using 20 µl collagen and was allowed to solidify at 37°C for at least 30 min before use. Note that each aortic piece were positioned flat on the surface of the collagen gel. After tissue placement, the gel bead and aortic piece were kept hydrated with EGM2-MV and care was taken to avoid completely submerging and dislodging the aorta piece from the bead. The explants were cultured at 37°C and 5% CO2 in an incubator and monitored daily. The key steps of primary MAEC isolation are summarized in Figure 1.
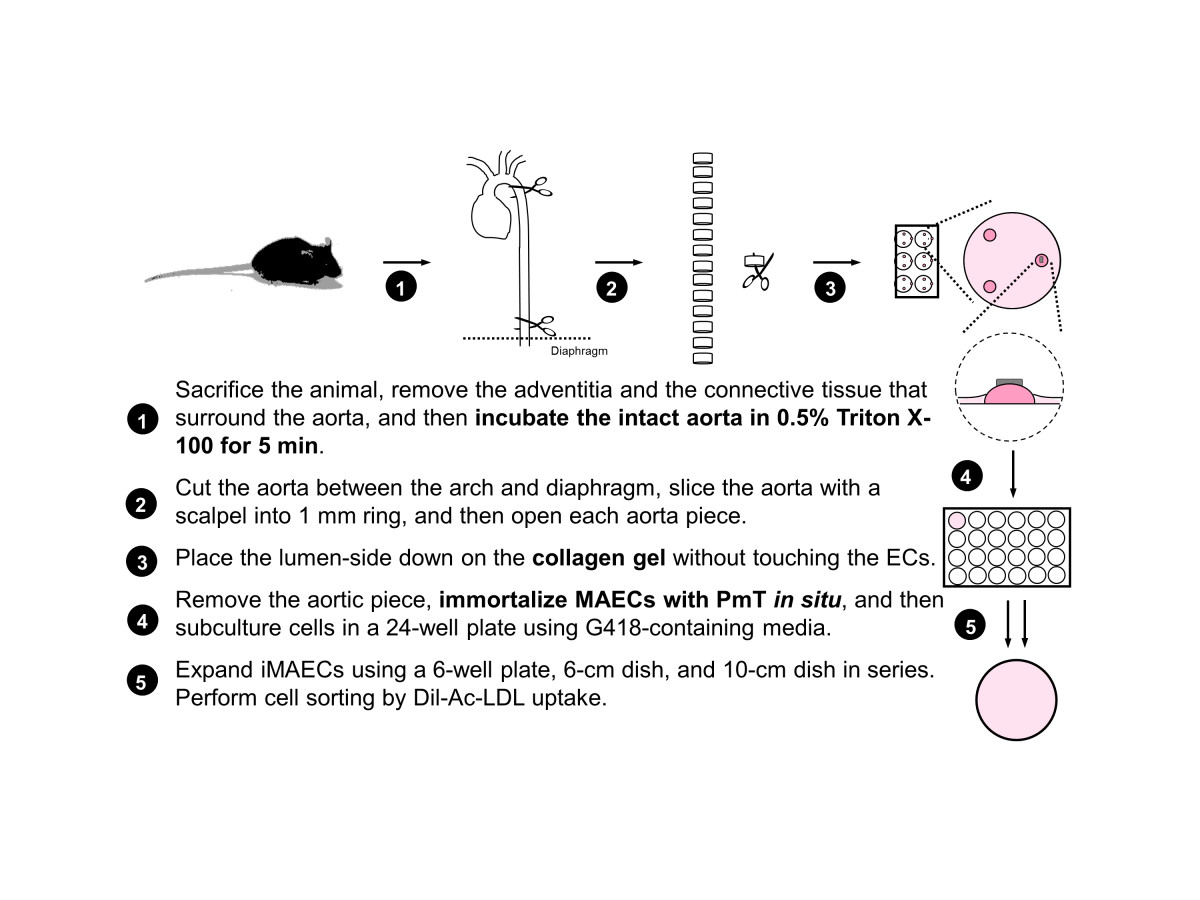
Figure 1
Figure 1 caption
Scheme of mouse aortic endothelial cell isolation and immortalization.
Immortalization
Since polyoma middle-sized T-antigen (PmT) is known to specifically immortalize endothelial cells [29, 30], we used the same method to immortalize MAEC as previously described by Balconi et al. to generate ECs from embryonic stem cells [31]. A PmT-producing packaging cell line was kindly provided by Dr. Elisabetta Dejana (Institute of Pharmacological Research, Milan, Italy). Briefly, PmT-conditioned medium was collected, filter sterilized using a 0.22 μm filter, and stored at -80°C until use. Once aortic cells began to grow out of the explants on the collagen gel, the aorta piece was removed within the next 3 to 4 days and complete growth medium (DMEM with 10% FBS, 1% endothelial cell growth supplement (ECGS) crude extract, 1% penicillin and streptomycin) was added. After one day of culture, MAEC were treated with the preserved PmT-conditioned medium, along with 8 μg/mL polybrene to increase infection efficiency (Sigma) for 4 hours at 37°C. After PmT incubation, the PmT-conditioned medium was removed and replaced with complete growth medium. 48 hours later, cells were passaged into a 24-well plate and grown in growth medium containing G418 (800 μg/mL) to select for immortalized cells containing the neomycin resistance gene. Cells were observed and passaged for several weeks (4 to 8 weeks) before complete cell selection was observed.
FACS cell sorting
Cells were stained with acetylated low density lipoprotein (Ac-LDL), labeled with 1,1′-dioctadecyl– 3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI-Ac-LDL, Biomedical Technologies) and then sorted by fluorescence-activated cell sorting. Briefly, cells were incubated with 10 μg/mL DiI-Ac-LDL for 4 hours at 37°C. Cells were then washed three times with fresh growth medium, trypsinized with 0.05% trypsin-EDTA, pelleted for 3 minutes at 2,300 RPM, and resuspended in 0.5-1.0 mL of sterile sorting buffer (1% FBS in 1X calcium- and magnesium-free HBSS). iMAEC were then sorted using a FACS Vantage SE (Becton Dickinson, San Jose, CA) using common gates for morphology (FSC-H vs SSC-H), singlets (FSC-W vs SSC-H), and separation gates for DiI staining. Since our goal was to collect pure iMAECs without any other contaminating cell types, we applied a highly stringent positive gating strategy by collecting only those cells that exhibited high DiI-LDL fluorescence intensity, which resulted in >95% DiI-positive cells post sorting. To effectively establish a stringent gating strategy, HUVECs and rat aortic smooth muscle cells (kindly provided by Dr. Kathy Griendling) were used as positive and negative controls, respectively. DiI-positive cells were collected in complete growth media and seeded on to a gelatin-coated (0.1%) culture dish. iMAEC were then observed and passaged at a ratio of 1 to 2 until characterization experiments.
Immunocytochemistry
Primary antibodies against PECAM-1 (Santa Cruz), VE-Cadherin (Cayman Chemical), von Willebrand Factor (WF) (Dako), and smooth muscle alpha actin (α-SMA, Sigma) were used for immunocytochemical staining of iMAEC and control cells, namely HUVEC as a positive control and 3T3 fibroblast and rat aortic smooth muscle cells (RASMC) as negative controls. Cells were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. Primary antibody diluted in 3% bovine serum albumin was applied overnight at 4°C, followed by incubation with a secondary antibody conjugated rhodamine red-X (Molecular Probes) for 1 hour at room temperature. Nuclei were labeled with Hoechst #33258 diluted in 3% bovine serum albumin for 15 min at room temperature. All cells were mounted using Dako mounting media (Dako), and fluorescence images were captured using fluorescence microscopy (Zeiss epi-fluorescent microscope).
Shear stress studies
iMAEC were grown to confluency in 100-mm tissue culture dishes (Falcon) and were subsequently exposed to laminar shear (LS, 15 dynes/cm2) or oscillatory shear (OS, ±5 dynes/cm2) using the cone-and-plate shear apparatus as previously described in our lab [32]. Early passages of iMAECs (passage # 6-10) and later passages of iMAECs (passage # 69-81) were also compared for their responses to shear stress. All shear stress studies were performed using complete growth medium for 24 h.
Endothelial tube formation assay
Following 24 h shear exposure, as described above, iMAECs were trypsinized and resuspended in 2% fetal bovine serum (FBS)-containing DMEM and 20,000 cells/well were added to a 96-well flat bottom plate coated with growth factor-reduced Matrigel (BD Biosciences). Following culture for 20 h, tubule formation was observed using a phase contrast microscope at 10× magnification. Tubule length was quantified using NIH ImageJ software [33].
Endothelial sprouting
Following 24 h shear exposure as described above, iMAECs were trypsinized, resuspended, and spotted at a density of 6 × 103 cells were embedding in a 1:1 mixture of DMEM and Matrigel and gently placed on the bottom of a 6-well plate. After polymerization at room temperature for 20 min, 2 mL of complete media was added. Endothelial sprouting was determined 2 days later by bright-field microscopy and counting the cells outside the bead periphery.
Scratch wound migration assay
A scratch-wound migration assay was performed using iMAECs previously exposed to either LS or OS for 24 h. Briefly, after shear, cell monolayers were scratched with a 200-μL pipette tip, media was replaced, and wounds were photographed at 0 and 6 hours [34]. NIH ImageJ software was used to quantify the closure of the wound over time by averaging six individual measurements of wound size for each wound at each time point. Results from three independent experiments performed in duplicate were pooled.
Western blotting
Following exposure to shear, cells were washed three times with ice-cold phosphate-buffered saline (PBS) and lysed with radioimmunoprecipitation assay buffer (RIPA) as described previously [35]. The lysate was further homogenized by sonication. The protein content of each sample was determined by Bio-Rad DC assays. Aliquots of cell lysate (20 μg of protein) were then resolved by size on 10% SDS-polyacrylamide gels and subsequently transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was incubated with a primary antibody overnight at 4°C, followed by incubation with an alkaline phosphatase-conjugated secondary antibody for 1 h at room temperature. Protein expression was detected using chemiluminescence, and the intensities of immunoreactive bands were determined via densitometry and the NIH ImageJ program [36]. Primary antibodies specific for KLF2, eNOS (BD Biosciences), β actin (Santa Cruz), VCAM-1 (Santa Cruz), Flk-1 (VEGF-R2, Santa Cruz), and Caveolin-1 (Santa Cruz) were used.
Quantitative real time PCR (qPCR)
Total RNA of each sample was reverse transcribed into cDNA using SuperScript III and random primers (Invitrogen) as previously described [37]. Briefly, qPCR was performed on selected genes using Brilliant II SYBR Green QPCR Master Mix (Stratagene) with custom-designed primers using a real-time PCR system (ABI StepOne Plus). All qPCR results were normalized based on 18S RNA expression.
Dihydroethidium (DHE) staining
iMAEC were stained in 2 μM DHE diluted in phosphate buffered saline for 30 minutes at 37°C. Cells were then fixed with 4% paraformaldehyde and mounted with DAKO mounting media and immediately imaged by fluorescence microscopy (Zeiss epi-fluorescent microscope).
Results
Morphology of cultured mouse aortic endothelial cells
The summary of the MAEC isolation and immortalization procedure is shown in Figure 1. During explant culture on collagen gel beads, MAEC migrated out of the aortic explants and gradually covered the gel within 3-4 days (Figure 2A, white arrows). When aortic explants were removed after 3-5 days of culture on the collagen bead, patches of endothelial cells were observed (Figure 2B, red arrows), suggesting that these are endothelial cells that did not migrate out (the red line indicates where the aortic explant was before it was removed) (Figure 2B). Both the migrating cells and the endothelial patches were cultured in a 24-well plate and immortalized by the polyoma middle T antigen method, which specifically immortalizes ECs [29, 30]. Immortalized cells were positively selected via G418 antibiotic over a 4 to 6 week period. To further ensure endothelial purity, we used a stringent gating strategy to collect only a portion of immortalized cells that were singlets and showed strong DiI-Ac-LDL fluorescence signal using HUVEC and RASM as positive and negative controls, respectively. Figure 2C-E show typical morphology of iMAEC obtained after the flow cytometric sorting using DiI-Ac-LDL.
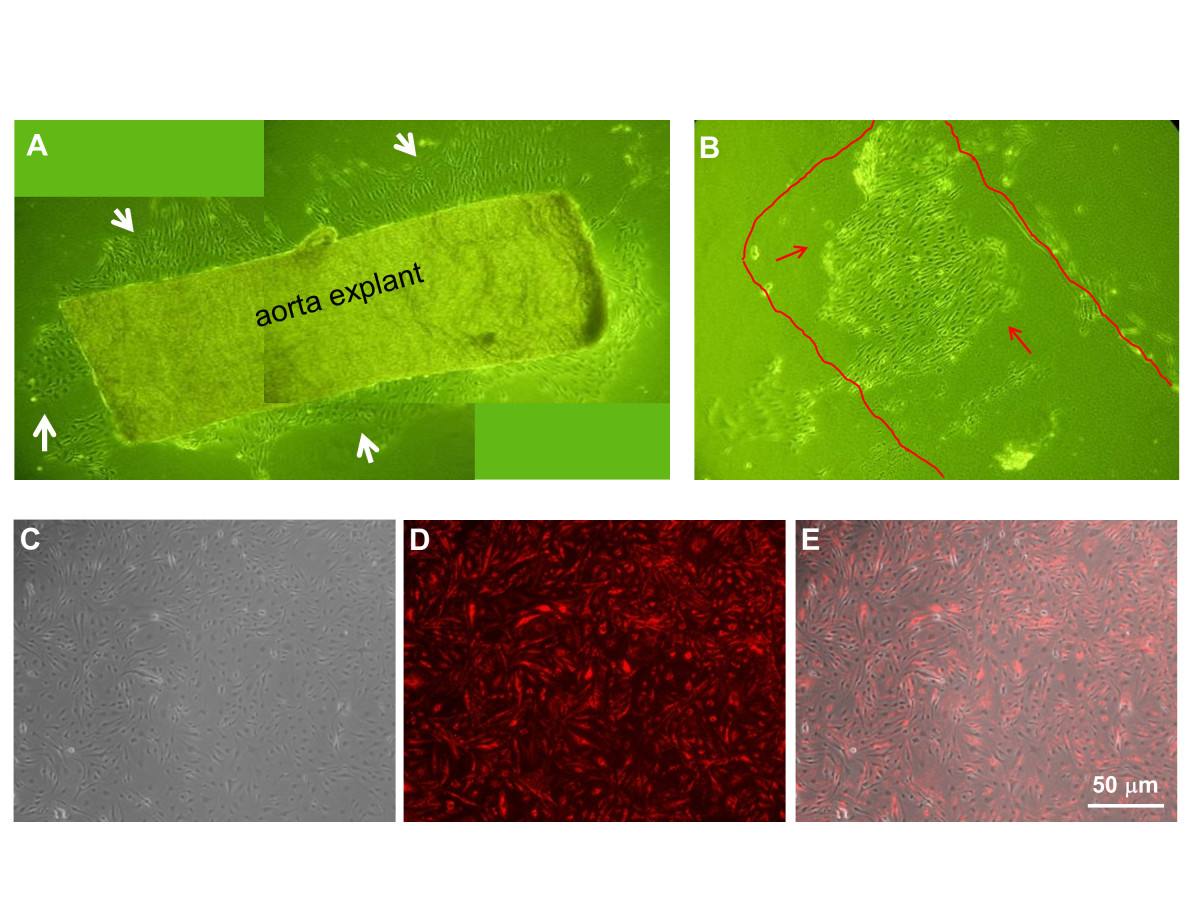
Figure 2
Figure 2 caption
Morphology of mouse aortic endothelial cells. (A) Aortic explants were cultured on top of collagen gel beads for 4 days. EC grew and migrated out of the aorta piece. (B) EC grown on collagen gel beads without migration seems to keep their original elongated morphology. (C-E) iMAECs collected after cell sorting were cultured for 24 h and imaged (C: phase contrast, D: DiI-Ac-LDL staining by fluorescence microscopy, and E: merged image). Scale bar = 50 μm.
Characterization of iMAECs
Using the described method, we developed several iMAEC lines, including wild-type (iMAEC-WT), Caveolin-1 knockout (iMAEC-cav1), eNOS knockout (iMAEC-eNOS), and p47phox knockout (iMAEC-p47). The phenotype of these immortalized cells was then examined. First, we performed Western blotting to confirm the lack of protein expression in knockout cell lines. As expected, all iMAEC and HUVEC expressed Flk1, while 3T3 and RASMC did not. Next, caveolin-1 in iMAEC-cav1 or eNOS in iMAEC-eNOS did not express cavleolin-1 or eNOS protein, respectively (Figure 3A). To further confirm the identity of these cell lines, we studied Dil-Ac-LDL uptake, as endothelial cells internalize and degrade Ac-LDL 7-15 times more efficiently than smooth muscle cells [38]. As shown in Figure 3B, iMAEC showed homogeneous Dil-Ac-LDL uptake similar to primary HUVEC. As expected, 3T3 fibroblasts and rat aorta smooth muscle cells (RASMC) failed to uptake Dil-Ac-LDL, demonstrating the staining specificity of Dil-Ac-LDL to endothelial cells. We also determined the shape-index of iMAECs to compare the morphology of these cells to HUVECs and primary MAECs. We found that iMAECs are more spindle-shaped compared to the other ECs such as HUVECs (Figure 3C), which may reflect maintenance of the cytoskeletal organization under high shear stress environment of mouse aortic ECs
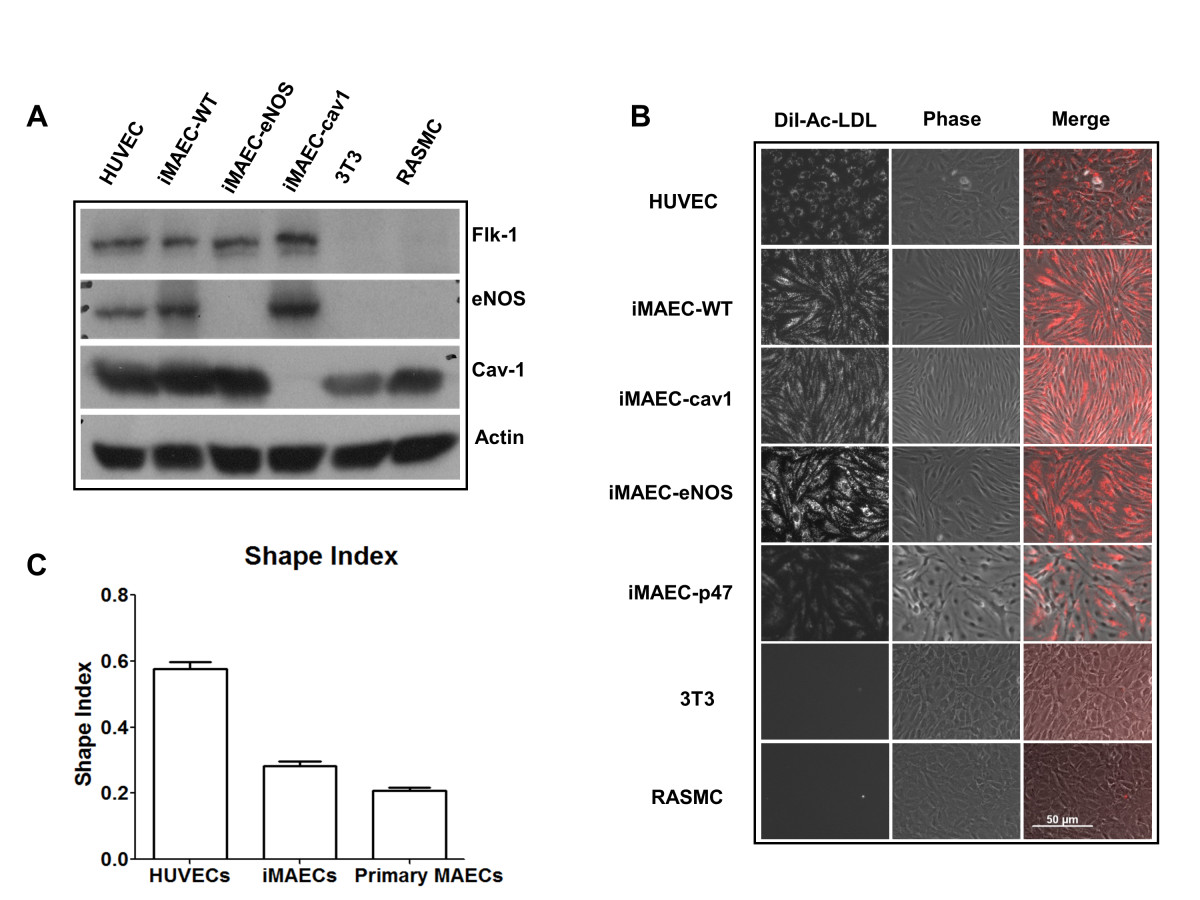
Figure 3
Figure 3 caption
Characterization of iMAEC lines by Dil-Ac-LDL staining. iMAEC lines including wild-type (iMAEC-WT), Caveolin-1 knockout (iMAEC-cav1), eNOS knockout (iMAEC-eNOS), and p47 knockout (iMAEC-p47), were cultured. (A) Total cell lysates were collected from iMAEC lines or control cells including HUVEC, 3T3, and RASMC. Western blotting was performed using specific antibodies against Flk-1, eNOS and Cav-1. Actin serves as an internal loading control. (B) iMAECs were incubated with Dil-Ac-LDL (10 μg/mL) for 4 h, and images were taken by fluorescence microscopy. HUVEC served as positive control while 3T3 and RASMC served as negative controls. Scale bar: 50 μm. (C) Graph shows the cell shape index of HUVECs, iMAECs and primary MAECs. For cell shape index calculation, 25 cells were chosen randomly from each group and analyzed by ImageJ software. Data represent means ± standard error.
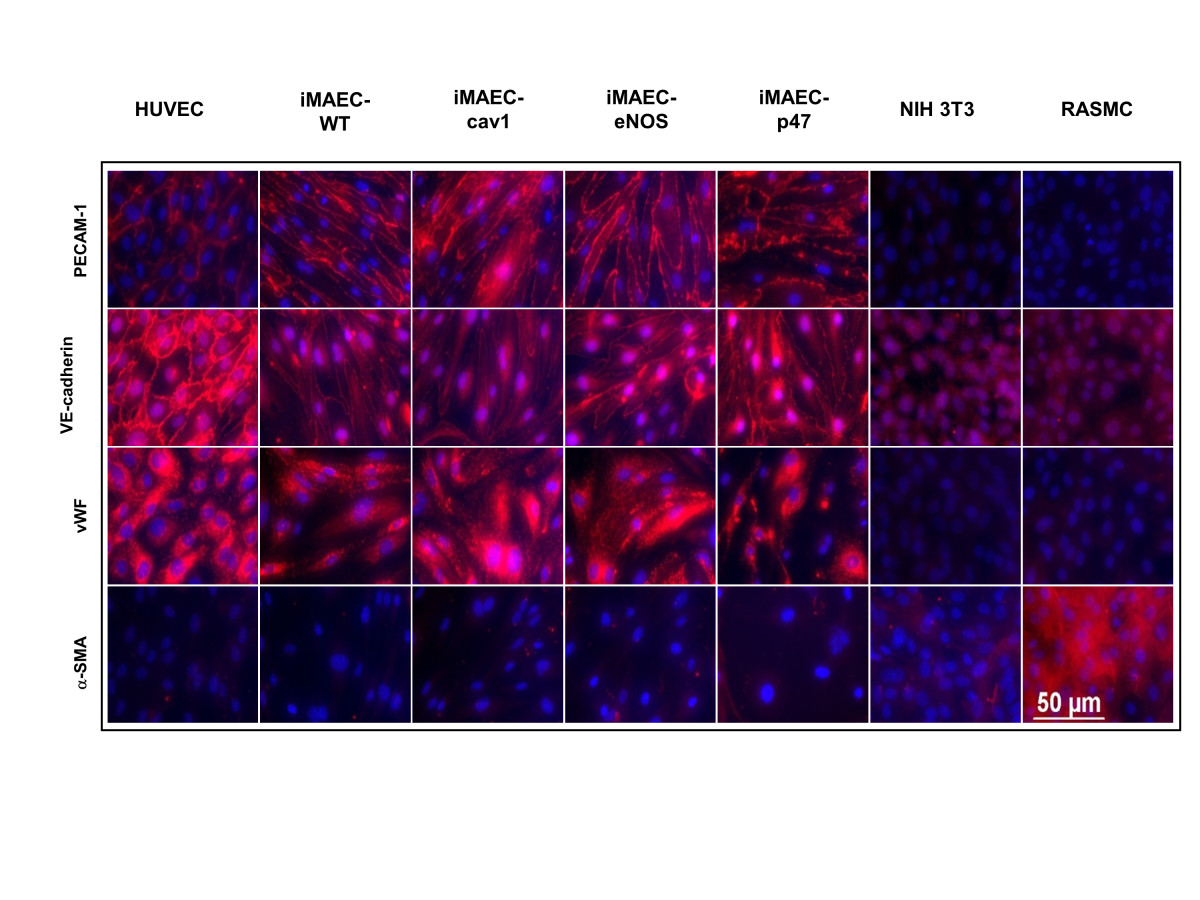
Figure 4
Figure 4 caption
Characterization of iMAEC lines by immunostaining. iMAEC lines including iMAEC-WT, iMAEC-cav1, iMAEC-eNOS, and iMAEC-p47 were immunostained using the endothelial markers, PECAM-1, VE-Cadherin, and von Willebrand factor (vWF). Smooth muscle cell α-actin (α-SMA) was used as negative marker. HUVEC served as a positive control while 3T3 and RASMC were negative controls. Scale bar = 50 μm.
iMAECs respond to shear stress
To further test whether iMAECs behave similarly to primary human EC cultures, we tested morphological and gene expression changes of these cells in response to shear stress. Like other cultured ECs, iMAECs exposed to unidirectional laminar shear stress (LS) aligned in the direction of the imposed flow, while those cultured under the oscillatory shear (OS) or static conditions did not. Further, it is well-known that LS induces expression of
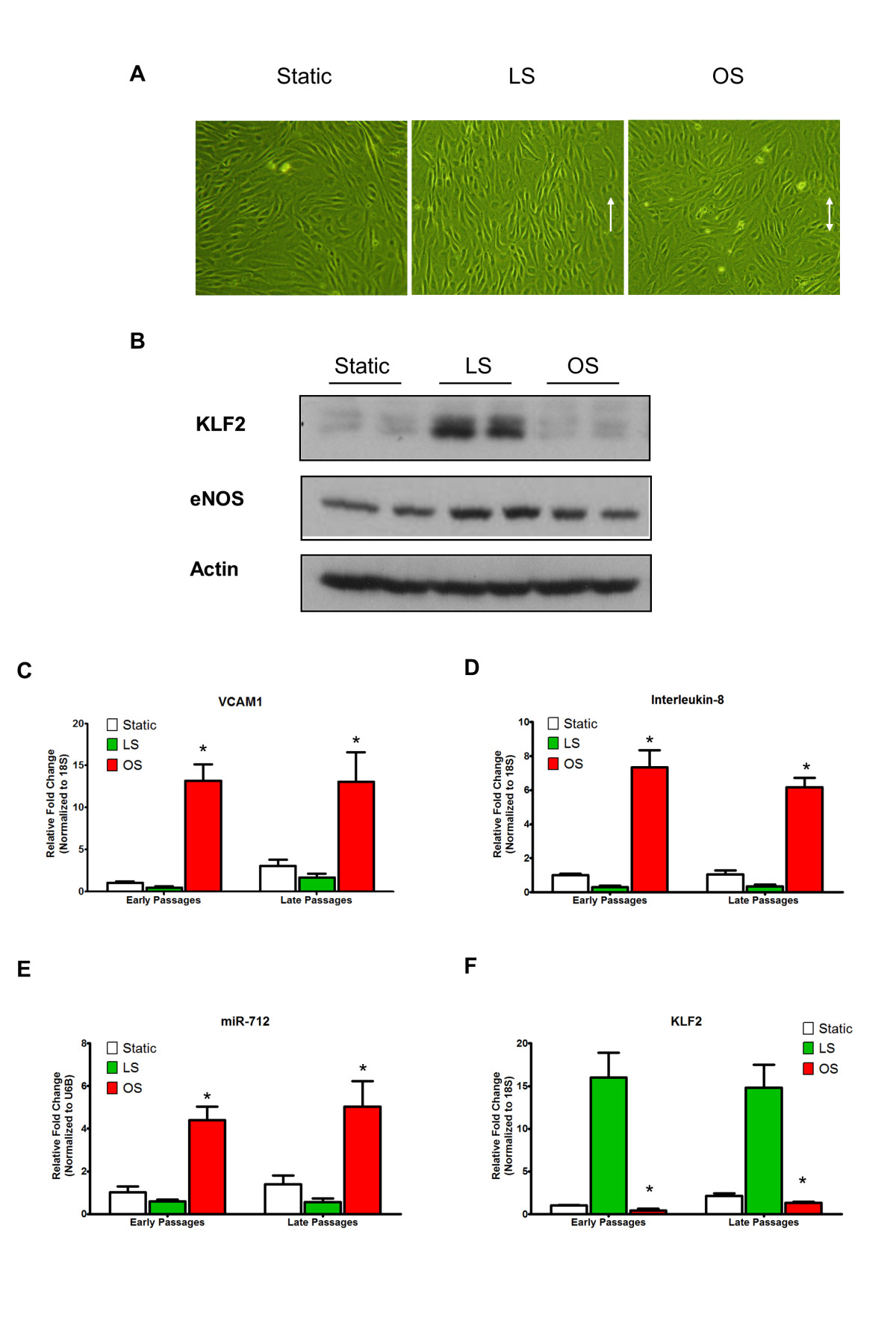
Figure 5
Figure 5 caption
iMAEC-WT maintains shear-sensitive endothelial phenotype. iMAEC-WT were exposed to LS or OS or kept for static for 24 h. (A) iMAEC-WT aligned in the direction of the flow when exposed to LS but not OS or static control. Arrows indicate the imposed flow direction. (B) Total cell lysates were collected and protein level of KLF2 and eNOS was measured by Western blot. Data were shown as means ± standard error, n = 3. (C-F) Expression of shear-sensitive genes and microRNA under LS and OS for 24 h using early and late passages of iMAEC cells. iMAECs from early and late passages were subjected to either LS or OS for 24 h and expression of , and (C, D, F) was determined by qPCR and normalized to 18S. (E) Graph shows the expression of shear-sensitive miRNA, miR-712 as determined by qPCR and normalized to RNU6B. Cells under static condition served as control. * p < 0.05 compared to respective LS controls.
Additionally, we have shown that OS induces endothelial tube formation and migration in various cultured ECs, including HUVEC [34, 41, 42]. Thus, we tested whether iMAECs respond to shear in a similar manner. As shown in Figure 6A-C, exposure of iMAECs to OS for 24 h induced endothelial tube formation, sprouting capability, and migration in the scratch wound-healing study, in comparison to LS and static conditions. These results suggest that iMAECs respond to shear stress in a manner similar to other cultured primary ECs.
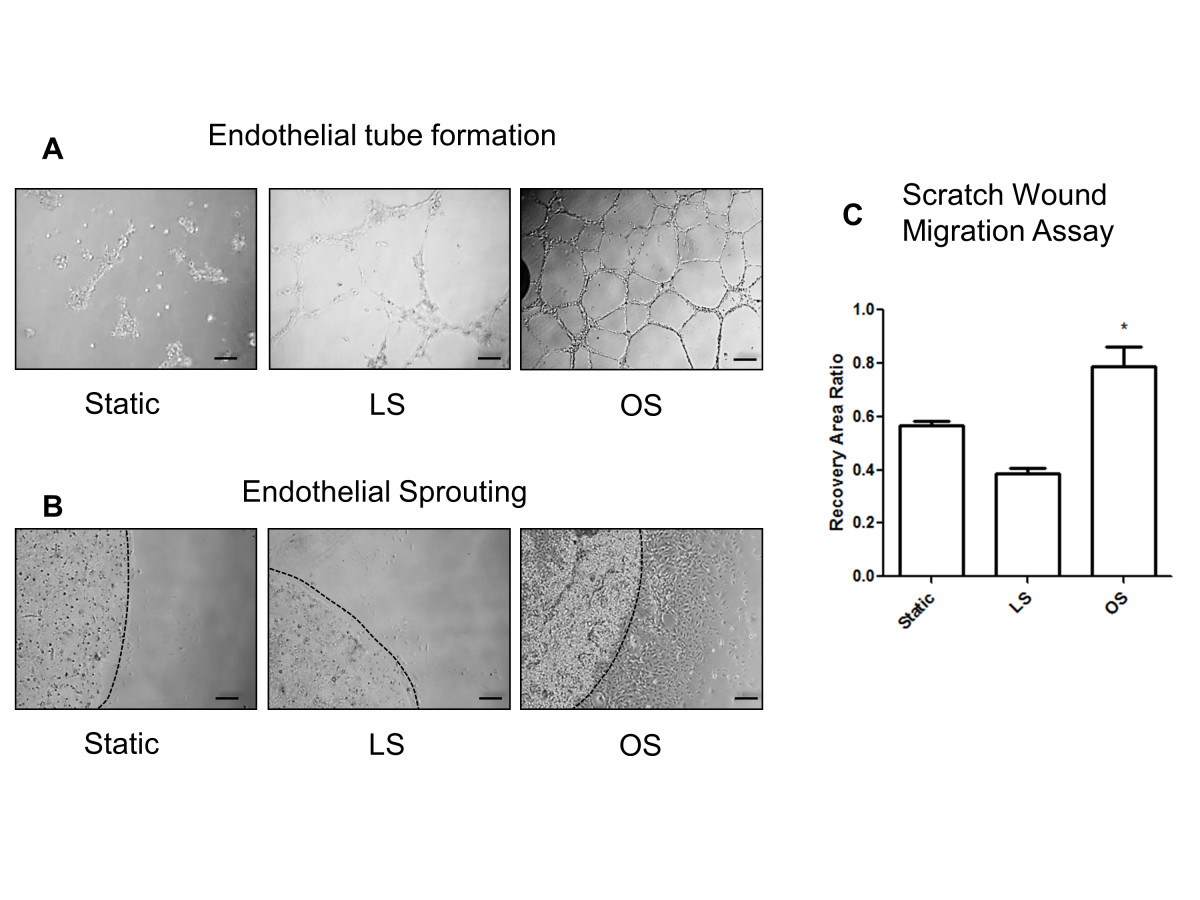
Figure 6
Figure 6 caption
Functional response of iMAECs to shear stress. (A) Endothelial cell tube formation; (B) endothelial cell sprouting; and (C) endothelial cell scratch wound migration assay performed using iMAECs exposed to LS or OS for 24 h. Dotted black line in B denotes the periphery of the Matrigel bead for sprouting assay. Black scale bar = 200 μm. Cells under static condition were used a controls. Data shown as means ± standard error; n = 3; * p < 0.05.
Next, we compared superoxide production between iMAEC-WT and iMAEC-p47phox Figure 7. Given that p47phox is an important component of NADPH oxidases, which produce superoxide, lack of p47 phox in EC is expected to reduce the production of superoxide [21]. Indeed, superoxide production, as demonstrated by dihydroethidium (DHE) staining, was significantly lower in iMAEC-p47 cells as compared to iMAEC-WT, thus both confirming the knockout efficiency in these cells as well as the role of p47phox in superoxide production.
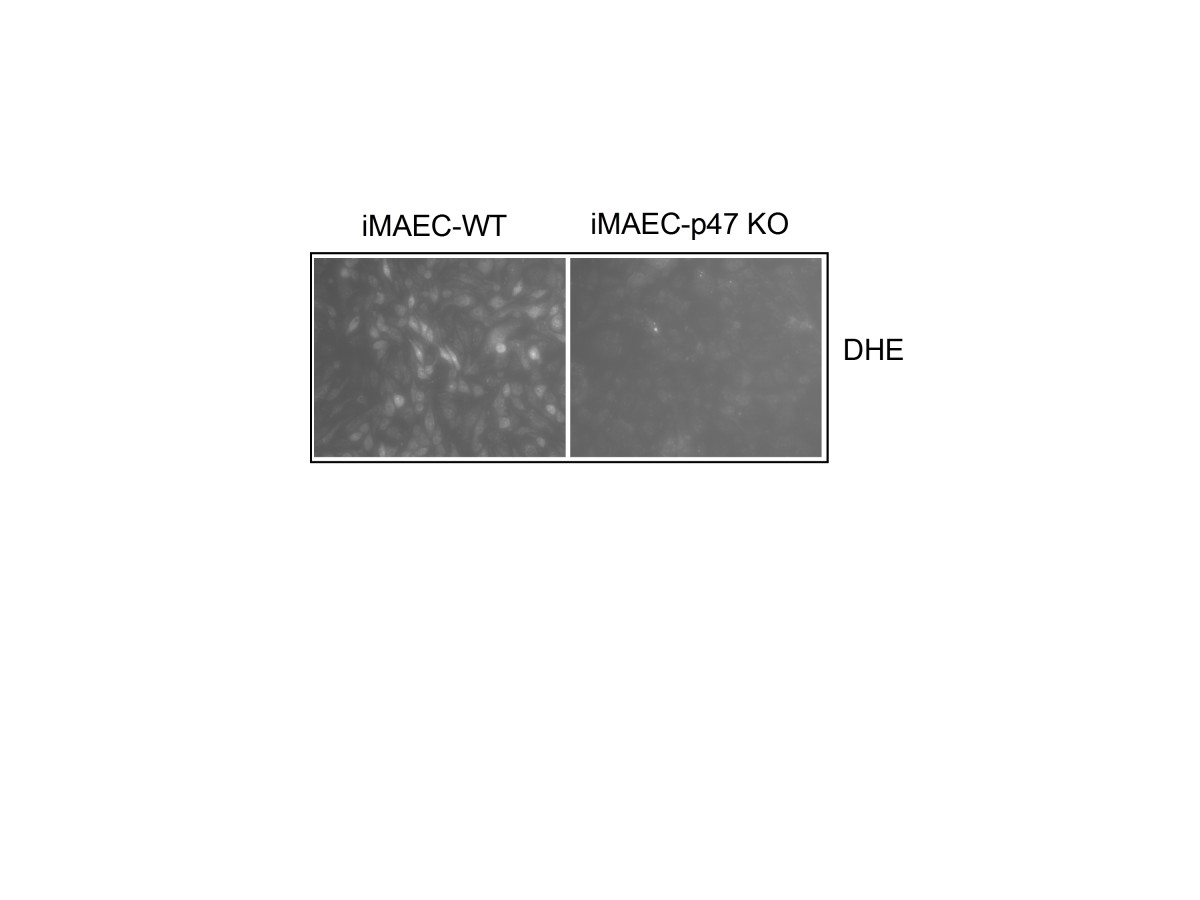
Figure 7
Figure 7 caption
VCAM-1 expression is elevated in iMAEC-eNOS while superoxide production is diminished in iMAEC-p47. iMAEC-WT and iMAEC-p47 were stained with DHE (2 μM) for 30 min and images were acquired using fluorescence microscopy.
Discussion
In this study, we developed an effective method to generate iMAEC lines that maintain an endothelial phenotype and respond to shear stress similar to other ECs, even after numerous passages. We also demonstrated functional differences between knockout and wild-type iMAEC lines in a gene-specific manner. For example, iMAEC-p47KO showed diminished production of superoxide. These results validate our MAEC isolation and immortalization protocol and provide a useful tool to study vascular biology. Given the vast array of transgenic mice, many unique iMAEC lines can be generated, expanded, and shared within research communities using this method.
Over the years, several groups have suggested different methods for the isolation and culture of primary MAEC [18, 19, 22–27]; however, the major issue of most protocols has been the difficulty in maintaining strict endothelial phenotype, as they are prone to transdifferentiation into non-endothelial phenotypes. In addition, it is difficult to obtain a large number of pure primary MAEC due to the small vessel size of mice. Moreover, characterization of primary endothelial cell cultures should be conducted regularly to confirm the lack of contamination or transdifferentiation. Previous reports have shown phenotypic change in cultured endothelial cells as a result of passaging [43]. It is difficult to maintain the phenotype of primary MAEC in sufficient amounts for a series of experiments without excessive time and effort. We compared iMAECs to HUVECs, which are the most widely used endothelial cells for
Importantly, immortalized cells are easily expandable and maintain an EC phenotype that for several months. It should also be noted that iMAEC lines only provide a model for studying vascular biology or disease
Explant culture of aortic tissue has been reported previously [18, 23, 25]. Most protocols used Matrigel as the base matrix for MAEC growth and migration [18, 23, 25]. Matrigel induces tube formation in ECs and has been widely used in angiogenesis studies. Matrigel may create an environment which stimulates cell proliferation and migration, potentially altering the phenotype of primary cells. Our method uses a collagen gel mixed with growth media, which has less of an effect on cellular phenotype, as demonstrated by our results To recapitulate, our results indicate that MAECs maintain their original morphology (Figure 2). Our modified method also provides a high yield of primary MAECs and they could be easily identified by their morphology, which is helpful when evaluating potential contamination of other cell types.
Immortalized mouse endothelial cells isolation from the embryo or brain in a variety of transgenic mice has been reported [31]. These studies demonstrated the need of transgenic iMAEC lines to address questions regarding the function of specific genes. To the best of our knowledge, this is the first report outlining a method to generate immortalized MAEC. As the origins of an EC, such as from an artery, vein, or microvessel, show different responses to stimuli, our method provides an adaptable protocol for developing EC lines from multiple locations.
Conclusion
Here, we developed a simple method to generate iMAEC lines, which maintain EC phenotype and functional responses to physiologically relevant shear stresses similar to primary ECs. Therefore, iMAECs can be used for multiple passages to study endothelial biology
Acknowledgements
The authors would like to thank Debra A. Smith for technical assistance in MAEC isolation and Dr. Elisabetta Dejana for providing us with the PmT lines. This work was supported by funding from NIH grants HL095070, HL114772, HL113451 to HJ. This work was also supported by the National Heart Lung and Blood Institute of the NIH as a Program of Excellence in Nanotechnology award HHSN268201000043C to HJ. SK is an American Heart Association Postdoctoral fellow. HJ thanks Ada Lee and Pete Correll and John and Jan Portman for the Professorships.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2
Authors’ original file for figure 3
Authors’ original file for figure 4
Authors’ original file for figure 5
Authors’ original file for figure 6
Authors’ original file for figure 7
References
- Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214:249-256.
- The Dynamic Response of Vascular Endothelial Cells to Fluid Shear Stress. J Biomech Eng. 1981;103:177-185.
- Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017-1022.
- Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234-247.
- Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745-2756.
- Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973;52:2757-2764.
- A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2:481-485.
- Human aortic endothelial cells express the type I but not the type II receptor for interleukin-1 (IL-1). J Cell Physiol. 1992;153:583-588.
- Isolation and characterization of human coronary artery-derived endothelial cells in vivo from patients undergoing percutaneous coronary interventions. J Vasc Res. 2009;46:487-494.
- Human dermal microvascular endothelial cells: an improved method for tissue culture and a description of some singular properties in culture. In Vitro Cell Dev Biol. 1985;21:627-635.
- Isolation, characterization and long-term culture of human myometrial microvascular endothelial cells. Hum Reprod. 2000;15:293-301.
- Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975;34:825-839.
- Interaction of aortic endothelial and smooth muscle cells in culture. Effect on glycosaminoglycan levels. Atherosclerosis. 1981;39:147-161.
- Functional analysis of an established mouse vascular endothelial cell line. J Vasc Res. 2007;44:138-148.
- Proinflammatory properties of murine aortic endothelial cells exclusively expressing a non cleavable form of TNFalpha. Effect on tumor necrosis factor alpha receptor type 2. Thromb Haemost. 2004;92:1428-1437.
- Regulation of endothelial glutathione by ICAM-1: implications for inflammation. FASEB J. 2004;18:1321-1323.
- Inhibition of endothelium-dependent vasorelaxation by extracellular K(+): a novel controlling signal for vascular contractility. Am J Physiol Heart Circ Physiol. 2004;286:H329-H339.
- An efficient, nonenzymatic method for isolation and culture of murine aortic endothelial cells and their response to inflammatory stimuli. In Vitro Cell Dev Biol Anim. 2003;39:43-50.
- In vitro culture and characterization of gene targeted mouse endothelium. Acta Physiol Scand. 2001;173:151-157.
- Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol. 2001;281:C1442-C1447.
- Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291-47298.
- Optimization of isolation and functional characterization of primary murine aortic endothelial cells. Endothelium. 2003;10:103-109.
- Characterisation of explanted endothelial cells from mouse aorta: electrophysiology and Ca2+ signalling. Pflugers Arch. 1999;438:612-620.
- A simple method for culturing mouse vascular endothelium. J Immunol Methods. 2001;254:31-45.
- Isolation of murine aortic endothelial cells in culture and the effects of sex steroids on their growth. In Vitro Cell Dev Biol Anim. 2003;39:140-145.
- “Lumen digestion” technique for isolation of aortic endothelial cells from heme oxygenase-1 knockout mice. Biotechniques. 2004;37:84-86.
- A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005;12:138-142.
- Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123-10128.
- Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci U S A. 1994;91:7291-7295.
- Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell. 1989;57:1053-1063.
- Development of endothelial cell lines from embryonic stem cells: A tool for studying genetically manipulated endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2000;20:1443-1451.
- Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid Redox Signal. 2011;15:1433-1448.
- NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671-675.
- Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2150-2156.
- Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622-1627.
- Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388-3396.
- Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535-H1543.
- Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034-2040.
- Prolonged shear stress and KLF2 suppress constitutive proinflammatory transcription through inhibition of ATF2. Blood. 2007;109:4249-4257.
- Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. J Biol Chem. 2005;280:12305-12309.
- The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000-.
- MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762-H1769.
- Human vascular endothelial cells with extended life spans: in vitro cell response, protein expression, and angiogenesis. Angiogenesis. 2002;5:21-33.
- Vascular Permeability Factor/Vascular Endothelial Cell Growth Factor-mediated Permeability Occurs through Disorganization of Endothelial Junctional Proteins. J Biol Chem. 1998;273:15099-15103.